Chestnut Oak Forest
- System
- Terrestrial
- Subsystem
- Forested Uplands
- State Protection
- Not Listed
Not listed or protected by New York State.
- Federal Protection
- Not Listed
- State Conservation Status Rank
- S4
Apparently Secure in New York - Uncommon in New York but not rare; usually widespread, but may be rare in some parts of the state; possibly some cause for long-term concern due to declines or other factors.
- Global Conservation Status Rank
- G5
Secure globally - Common in the world; widespread and abundant (but may be rare in some parts of its range).
Summary
Did you know?
Chestnut oak is one of the important trees, along with sugar maple and red oak, that replaced American chestnut after the devastating spread of chestnut blight. The American chestnut was a common component in chestnut oak forests prior to the chestnut blight in the early 1900s. The blight was caused by an introduced Asian fungus (Cryphonectria parasitica) and by 1950, the once common chestnut was reduced to decomposing logs and small stump sprouts.
State Ranking Justification
There are several hundred occurrences statewide. Some documented occurrences have good viability and many are protected on public land or private conservation land. This community has a somewhat limited distribution in the state and includes several very large, high quality examples. The current trend of this community is probably stable for occurrences on public land, or declining slightly elsewhere due to moderate threats related to development pressure.
Short-term Trends
The number and acreage of chestnut oak forests in New York has probably declined slightly in recent decades as a result of fire suppression, logging, fragmentation, and other development. Larger occurrences may continue to undergo fragmentation. Fire suppression may promote mesification of some occurrences with the result of succession to another community. However, historical reforestation of much of southeastern New York may have resulted in an increase in this community in recent history. Thus, although decline is expected in the long term, this community may be relatively stable in the short term.
Long-term Trends
The number and acreage of chestnut oak forests in New York have probably declined substantially from historical numbers likely correlated to fire suppression, fragmentation, and other development. Continuing unnatural simplification or conversion of the forest's composition, structure, and age class distribution may also threaten the community's integrity in the long term.
Conservation and Management
Threats
Threats to forests in general include changes in land use (e.g., clearing for development), forest fragmentation (e.g., roads), and invasive species (e.g., insects, diseases, and plants). Other threats may include over-browsing by deer, fire suppression, and air pollution (e.g., ozone and acidic deposition). When occurring in expansive forests, the largest threat to the integrity of chestnut oak forests are activities that fragment the forest into smaller pieces. These activities, such as road building and other development, restrict the movement of species and seeds throughout the entire forest, an effect that often results in loss of those species that require larger blocks of habitat (e.g., black bear, bobcat, birds). Additionally, fragmented forests provide decreased benefits to neighboring societies from services these societies often substantially depend on (e.g., clean water, mitigation of floods and droughts, pollination in agricultural fields, and pest control) (Daily et al. 1997).Over time, the dry, rocky slopes most appropriate for this community can become invaded by plants that slowly enrich the site if fire or other disturbances are suppressed. Such enrichment, or "mesification" is also a threat to this community. Chestnut oak forests are threatened by development (e.g., residential, agricultural, communication antennas), either directly within the community or in the surrounding landscape. Other threats include habitat alteration (e.g., roads, mining, deer over-browsing, trash dumping), and recreational overuse (e.g., ATVs, hiking trails, campgrounds, horseback riding, mountain biking). Loss of the natural range of variability and pattern of canopy closure from unnatural canopy removal and the accompanying ground disturbance may be a threat to the integrity of the community. A few chestnut oak forests are threatened by invasive species, such as multiflora rose (Rosa multiflora). Suppression of the natural fire regime may also be a threat to this community.Chestnut oak forests may be threatened by the non-native spongy moth (Lymantria dispar), which is one of the most devastating forest pests in North America . The spongy moth is known to feed on the foliage of hundreds of species of plants in North America but its most common hosts are oaks and aspen. Spongy moth populations are typically eruptive in North America; in any forest stand densities may fluctuate from near 1 egg mass per ha to over 1,000 per ha. When densities reach very high levels, trees may become completely defoliated. Several successive years of defoliation, along with contributions by other biotic and abiotic stress factors, may ultimately result in tree mortality (McManus et al. 1980, Liebold 2003).
Conservation Strategies and Management Practices
Management should focus on activities that help maintain regeneration of the species associated with this community. Deer have been shown to have negative effects on forest understories (Miller et al. 1992, Augustine and French 1998, Knight 2003) and management efforts should strive to ensure that regenerating trees and shrubs are not so heavily browsed that they cannot replace overstory trees. Additionally, dry rocky communities such as this one are showing a trend in being slowly replaced by more shade-tolerant, richer-soil species such as sugar maple and more often non-native species such as Norway maple and tree of heaven. This trend towards "mesification" is likely a result of the elimination of fire as a natural process in this forest system. Periodic forest fires used to expose bedrock, remove excess organic material, and open up gaps in the forest canopy. With fire suppression, dry, relatively open and rocky forest communities such as the chestnut oak forest are slowly being replaced. Management for these communities should consider this phenomenon.
Development and Mitigation Considerations
Strive to minimize fragmentation of large forest blocks by focusing development on forest edges, minimizing the width of roads and road corridors extending into forests, and designing cluster developments that minimize the spatial extent of the development. Development projects with the least impact on large forests and all the plants and animals living within these forests are those developments built on brownfields or other previously developed land. These projects have the added benefit of matching sustainable development practices (for example, see: The President's Council on Sustainable Development 1999 final report, US Green Building Council's Leadership in Energy and Environmental Design certification process at http://www.usgbc.org/).
Inventory Needs
Inventory any remaining large and/or old-growth examples across the state. Continue searching for large sites in excellent to good condition (A- to AB-ranked).
Research Needs
Regularly assess the presence and degree of impact that spongy moth has on this forest community. Determine the optimal fire regime for this community.
Rare Species
- Atrytonopsis hianna (Dusted Skipper) (guide)
- Calamagrostis porteri ssp. porteri (Porter's Reed Grass) (guide)
- Callitriche terrestris (Terrestrial Water Starwort) (guide)
- Carex glaucodea (Glaucous Sedge) (guide)
- Carex nigromarginata (Black-edged Sedge) (guide)
- Carphophis amoenus (Eastern Wormsnake) (guide)
- Catocala herodias gerhardi (Herodias or Pine Barrens Underwing) (guide)
- Celastrina neglectamajor (Appalachian Azure) (guide)
- Citheronia regalis (Regal Moth) (guide)
- Crotalus horridus (Timber Rattlesnake) (guide)
- Endodeca serpentaria (Virginia Snakeroot) (guide)
- Geothlypis formosa (Kentucky Warbler) (guide)
- Glena cognataria (Blueberry Gray) (guide)
- Haliaeetus leucocephalus (Bald Eagle) (guide)
- Hemileuca maia maia (Inland Barrens Buckmoth) (guide)
- Malaxis bayardii (Bayard's Adder's Mouth Orchid) (guide)
- Myotis leibii (Eastern Small-footed Myotis) (guide)
- Myotis lucifugus (Little Brown Bat) (guide)
- Myotis septentrionalis (Northern Long-eared Bat) (guide)
- Neotoma magister (Allegheny Woodrat) (guide)
- Oxalis violacea (Violet Wood Sorrel) (guide)
- Platanthera hookeri (Hooker's Orchid) (guide)
- Pseudotriton ruber (Red Salamander) (guide)
- Sceloporus undulatus (Fence Lizard) (guide)
- Setophaga cerulea (Cerulean Warbler) (guide)
- Speranza exonerata (Barrens Itame) (guide)
- Sympistis dentata (Toothed Apharetra) (guide)
- Zale curema (Black-eyed Zale) (guide)
- Zanclognatha martha (Pine Barrens Zanclognatha) (guide)
Range
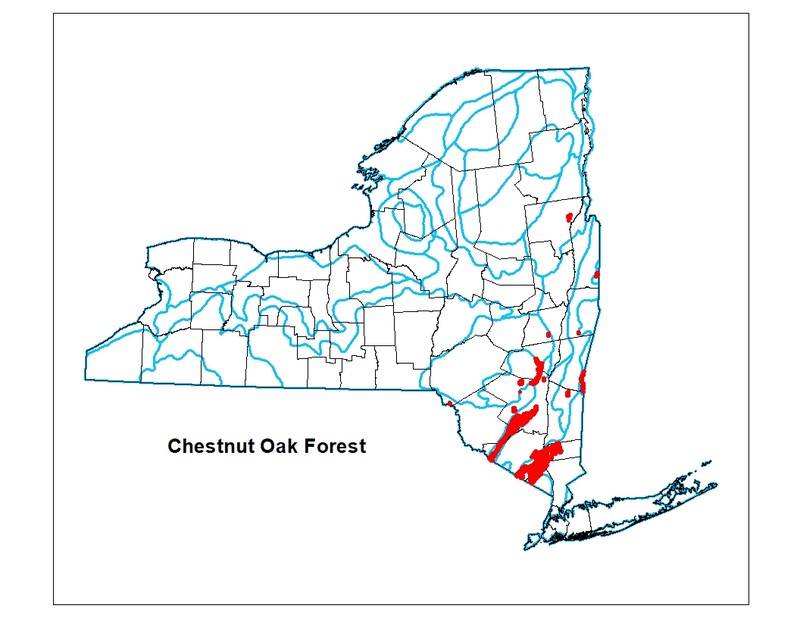
New York State Distribution
This community is currently known from the lower Hudson Valley where it is most common in the Hudson Highlands and Shawangunk Mountains. Chestnut oak forests are also known from the Catskill Mountains, the Rensselaer Plateau, and it reaches its northern limit in the Lake George Valley.
Global Distribution
The range of this community is estimated to span northeast to southern Maine, west to Ohio and Kentucky, south to Alabama and Georgia and southeast to North Carolina and Maryland.
Best Places to See
- Minnewaska State Park (Ulster County)
- Clarence-Fahnstock State Park (Putnam County)
- Bear Mountain State Park (Orange, Rockland Counties)
- Taconic State Park
- Harriman State Park (Orange, Rockland Counties)
Identification Comments
General Description
A hardwood forest that occurs on well-drained and often rocky sites in glaciated portions of the Appalachians and on the coastal plain. The combined cover of chestnut, red, white, and black oak should generally exceed 25%. Typically, there is more chestnut oak than hickories. The understory may vary, with high abundance of mountain laurel, huckleberry, or Pennsylvania sedge. At least three edaphic variants with different understory dominants are known: 1) a tall shrub-dominated understory with 60-90% mountain laurel, 2) a short shrub-dominated understory with dense dwarf heaths, such as black huckleberry, and 3) a herb-dominated understory with Pennsylvania sedge.
Characters Most Useful for Identification
Dominant trees are typically chestnut oak (Quercus montana) and red oak (Q. rubra). Common associates are white oak (Q. alba), black oak (Q. velutina), and red maple (Acer rubrum). American chestnut (Castanea dentata) was a common associate in these forests prior to the chestnut blight; chestnut sprouts are still found in some stands. The shrublayer is predominantly ericaceous; characteristic shrubs are black huckleberry (Gaylussacia baccata), mountain laurel (Kalmia latifolia), and blueberry (Vaccinium pallidum). Common groundlayer plants are Pennsylvania sedge (Carex pensylvanica), wild sarsaparilla (Aralia nudicaulis), wintergreen (Gaultheria procumbens), and cushions of the moss Leucobryum glaucum.
Elevation Range
Known examples of this community have been found at elevations between 100 feet and 4,000 feet.
Best Time to See
At sites with abundant mountain laurel, a great time to see this community is when mountain laurel is flowering in early summer.
Chestnut Oak Forest Images
Classification
International Vegetation Classification Associations
This New York natural community encompasses all or part of the concept of the following International Vegetation Classification (IVC) natural community associations. These are often described at finer resolution than New York's natural communities. The IVC is developed and maintained by NatureServe.
- Chestnut Oak - (White Oak, Scarlet Oak) / Mapleleaf Viburnum - (Mountain Laurel) Forest (CEGL005023)
- Chestnut Oak - Northern Red Oak / American Witch-hazel Forest (CEGL006057)
- Chestnut Oak - (Northern Red Oak, Black Oak) / (Lowbush Blueberry, Blue Ridge Blueberry) Forest (CEGL006282)
- Northern Red Oak - (Chestnut Oak) / Blueberry species / Wavy Hairgrass Woodland (CEGL006134)
NatureServe Ecological Systems
This New York natural community falls into the following ecological system(s). Ecological systems are often described at a coarser resolution than New York's natural communities and tend to represent clusters of associations found in similar environments. The ecological systems project is developed and maintained by NatureServe.
- Central Appalachian Dry Oak-Pine Forest (CES202.591)
- Northeastern Interior Dry-Mesic Oak Forest (CES202.592)
Characteristic Species
-
Trees > 5m
- Acer rubrum var. rubrum (common red maple)
- Betula lenta (black birch)
- Quercus alba (white oak)
- Quercus montana (chestnut oak)
- Quercus rubra (northern red oak)
-
Shrubs 2 - 5m
- Hamamelis virginiana (witch-hazel)
- Kalmia latifolia (mountain laurel)
-
Shrubs < 2m
- Gaylussacia baccata (black huckleberry)
- Vaccinium angustifolium (common lowbush blueberry)
- Vaccinium pallidum (hillside blueberry)
-
Vines
- Parthenocissus quinquefolia (Virginia-creeper)
-
Herbs
- Arisaema triphyllum ssp. triphyllum (common jack-in-the-pulpit)
- Avenella flexuosa (common hair grass)
- Carex pensylvanica (Pennsylvania sedge)
- Eurybia divaricata (white wood-aster)
Similar Ecological Communities
- Allegheny oak forest
(guide)
Chestnut oak forest has fewer canopy dominants and a less diverse shrublayer and groundlayer flora.
- Appalachian oak-hickory forest
(guide)
Chestnut oak forest tends to lack hickories and hop hornbeam. More specifically, the combined cover of chestnut oak forest indicator species (Quercus montana, Vaccinium pallidum, Gaultheria procumbens, Gaylussacia baccata, and Kalmia latifolia) exceed Appalachian oak-hickory forest indicators (Carya spp., Ostrya virginiana, Fraxinus americana, Cornus florida, Viburnum rafinesquianum, V. acerifolium, Amelanchier spp., and Rhus aromatica). Chestnut oak forest seems to occur more often on acidic bedrock in contrast to circumneutral bedrock in Appalachian oak-hickory forest.
- Coastal oak-hickory forest
(guide)
Chestnut oak forest occurs over bedrock and large rocks (instead of sand and gravel), and has higher abundances of chestnut oak (instead of scarlet oak).
- Coastal oak-laurel forest
(guide)
Coastal oak-laurel forest is dominated by scarlet oak (Quercus coccinea), has a lower abundance of chestnut oak, and has no red oak. Chestnut oak forests are strongly dominated by chestnut oak and red oak.
Vegetation
Percent cover
This figure helps visualize the structure and "look" or "feel" of a typical Chestnut Oak Forest. Each bar represents the amount of "coverage" for all the species growing at that height. Because layers overlap (shrubs may grow under trees, for example), the shaded regions can add up to more than 100%.
Additional Resources
References
Augustine, A.J. and L.E. French. 1998. Effects of white-tailed deer on populations of an understory forb in fragmented deciduous forests. Conservation Biology 12:995-1004.
Cain, S.A. 1936. The composition and structure of an oak woods, Cold Spring Harbor, Long Island, with special attention to sampling methods. American Midland Naturalist 19: 390-416.
Conard, H.S. 1935. The plant associations of central Long Island. American Midland Naturalist 16:433-515.
Daily, G.C., S. Alexander, P.R. Ehrlich, L. Goulder, J. Lubchenco, P. Matson, H.A. Mooney, S. Postel, S.H. Schneider, D. Tilman, and G.M. Woodwell. 1997. Ecosystem Services: benefits supplied to human societies by natural ecosystems. Issues In Ecology 2:1-16.
Edinger, G. J., D. J. Evans, S. Gebauer, T. G. Howard, D. M. Hunt, and A. M. Olivero (editors). 2014. Ecological Communities of New York State. Second Edition. A revised and expanded edition of Carol Reschke’s Ecological Communities of New York State. New York Natural Heritage Program, New York State Department of Environmental Conservation, Albany, NY. https://www.nynhp.org/ecological-communities/
Edinger, Gregory J., D.J. Evans, Shane Gebauer, Timothy G. Howard, David M. Hunt, and Adele M. Olivero (editors). 2002. Ecological Communities of New York State. Second Edition. A revised and expanded edition of Carol Reschke's Ecological Communities of New York State. (Draft for review). New York Natural Heritage Program, New York State Department of Environmental Conservation. Albany, NY. 136 pp.
Eyre, F.H., ed. 1980. Forest cover types of the United States and Canada. Society of American Foresters, Washington, D.C.
Greller, Andrew M. 1977. A classification of mature forests on Long Island, New York. Bull. Torrey Bot. Club 140 (4):376-382.
Hagan, J.M. and A.A. Whitman. 2004. Late-successional Forest:A disappearing age class and implications for biodiversity. Manomet Center for Conservation Sciences. 4 pp.
Knight, T.M. 2003. Effects of herbivory and its timing across populations of Trillium grandiflorum (Liliaceae). American Journal of Botany 90:1207-1214.
Liebold, S. 2003. Gypsy Moth in North America. U.S. Department of Agriculture, Forest Service, Northeastern Research Station, Morgantown, WV.
McIntosh, R.P. 1972. Forests of the Catskill Mountains, New York. Ecol. Monogr. 42:143-161.
McManus, M, N. Schneebergerm R. Reardon, and G. Mason. 1980. Gypsy Moth. Forest Insect and Disease Leaflet 162. U.S. Department of Agriculture, Forest Service, Washington, D.C.
McVaugh, R. 1958. Flora of the Columbia County area, New York. Bull. 360. New York State Museum and Science Service. University of the State of New York. Albany, NY. 400 pp.
Miller, S.G., S.P. Bratton, and J. Hadidian. 1992. Impacts of white-tailed deer on endangered and threatened vascular plants. Natural Areas Journal 12:67-74.
New York Natural Heritage Program. 2024. New York Natural Heritage Program Databases. Albany, NY.
Reschke, Carol. 1990. Ecological communities of New York State. New York Natural Heritage Program, New York State Department of Environmental Conservation. Latham, NY. 96 pp. plus xi.
Ross, P. 1958. Microclimatic and vegetational studies in a cold-wet deciduous forest. Black Rock Forest Papers No. 24, Harvard Black Rock Forest, Cornwall-on-the-Hudson, New York.
The President's Council on Sustainable Development. 1999. Towards a Sustainable America: Advancing Prosperity, Opportunity, and a Healthy environment for the 21st Century. Washington, DC. 97 pp. plus appendices.
Links
About This Guide
Information for this guide was last updated on: December 13, 2023
Please cite this page as:
New York Natural Heritage Program. 2024.
Online Conservation Guide for
Chestnut oak forest.
Available from: https://guides.nynhp.org/chestnut-oak-forest/.
Accessed July 26, 2024.